


An MM Expert Shares New Developments
Hear Dr Sikander Ailawadhi, Hematologist-Oncologist, discuss multiple myeloma, including unmet need and targets under investigation.

DISEASE STATE
Multiple myeloma (MM) originates in the bone marrow and secondary lymphoid organs, where B cells undergo differentiation before transforming into plasma cells.1 Mutations occur when B cells differentiate and lead to the formation of malignant plasma cells that produce an abnormal amount of nonfunctional antibodies (M-protein).1,2
Learn about MM, including changes to the treatment landscape.
Multiple myeloma prevalence3,4
Multiple myeloma is the third most common hematologic cancer. In 2023, it is estimated that there will be 35,730 new cases and 12,590 deaths in the U.S.
Multiple myeloma is most frequently diagnosed among patients aged 65-74 years.
Median age
at diagnosis is
69 years
Higher incidences in Black and male patients

Diagnostic criteria in multiple myeloma
IMWG CONSIDERS CLONAL BONE MARROW PLASMA CELLS ≥10% OR BIOPSY-PROVEN BONY OR EXTRAMEDULLARY PLASMACYTOMA* AND ANY 1 OR MORE OF THE FOLLOWING MYELOMA-DEFINING EVENTS5†
CRAB
- C = Hypercalcemia
- R = Renal insufficiency
- A = Anemia
- B = Bone/skeletal-related events (pathological fractures)
SLiM
- S = Clonal plasma cells in bone marrow ≥60%*
- Li = Involved vs uninvolved serum free light chain ratio ≥100‡
- M = >1 MRI focal lesion§
*Clonality should be established by showing κ/λ-light-chain restriction on flow cytometry, immunohistochemistry, or immunofluorescence. Bone marrow plasma cell percentage should preferably be estimated from a core biopsy specimen; in case of a disparity between the aspirate and core biopsy, the highest value should be used.
†The International Myeloma Working Group (IMWG) recommends using both CRAB symptoms and SLiM biomarkers for diagnosis.
‡These values are based on the serum Freelite assay (The Binding Site Group, Birmingham, UK). The involved free light chain must be ≥100 mg/L.
§Each focal lesion must be 5 mm or more in size.
Multiple myeloma is a genetically complex disease and includes both asymptomatic (monoclonal gammopathy of undetermined significance [MGUS], smoldering multiple myeloma [SMM]) and symptomatic (active myeloma) stages.6-9
Common mutations in multiple myeloma include6:
- Translocations
- Hyperdiploidy
- Deletions and amplifications of chromosome arms
- Ras mutations
As Multiple Myeloma progresses, the potential for mutations increases.6

Unmet Need
Multiple myeloma remains an incurable disease, with nearly all patients relapsing and requiring subsequent therapy1,10-17

- With each subsequent relapse, fewer treatment options remain18,19
- Each remission is typically shorter than the previous one1
PATIENTS FACE A REDUCTION IN TREATMENT RESPONSE WITH SUBSEQUENT LINES OF THERAPY20
The MAMMOTH study was a multicenter, retrospective study conducted from January 2017 to June 2018, which included 275 patients with MM. Study objective was to investigate the natural history and outcomes of patients with MM refractory to CD38 monoclonal antibodies.
In the study, patients progressed on multiple lines of treatment (including proteasome inhibitors, immunomodulatory agents, or high-dose alkylating chemotherapy) and were refractory to CD38 monoclonal antibodies.
- For triple- and quad-refractory patients on the next line of therapy, overall response rate (ORR) was 29%
- At their fifth line of therapy or further, ORR declined to 18%
SURVIVAL RATES CAN DECLINE AS PATIENTS BECOME REFRACTORY TO MORE THERAPIES20
In the MAMMOTH study, median overall survival (mOS) in triple/quad refractory was <10 months20
- Not triple-refractory (N=57) mOS=11.2 months (95% CI: 5.4-17.1)
- Triple- and quad-refractory (N=148) mOS=9.2 months (95% CI: 7.1-11.2)
- Penta-refractory (N=70) mOS=5.6 months (95% CI: 3.5-7.8)

Current Treatment Classes
There are currently three primary classes of treatment in multiple myeloma18,21,22
Inhibitors
Agents
Monoclonal
Antibodies
OTHER APPROVED CLASSES INCLUDE: ANTI-SLAMF7, SINE, AND ANTI-BCMAs23-28
Many surface proteins are differentially expressed on multiple myeloma cells compared to healthy cells.22,29-34

Investigational Targets
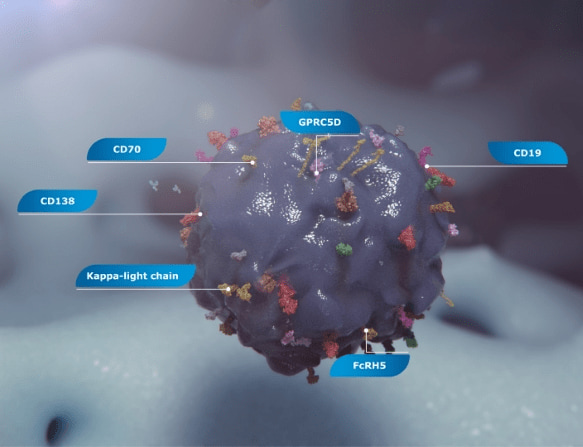
There are also several surface protein treatment targets under investigation in multiple myeloma, including CD138, FcRH5, CD19, GPRC5D, κ-light chain, and CD7029,31-34
Two protein targets that are furthest along in their investigation35,36:
GPRC5D37,38
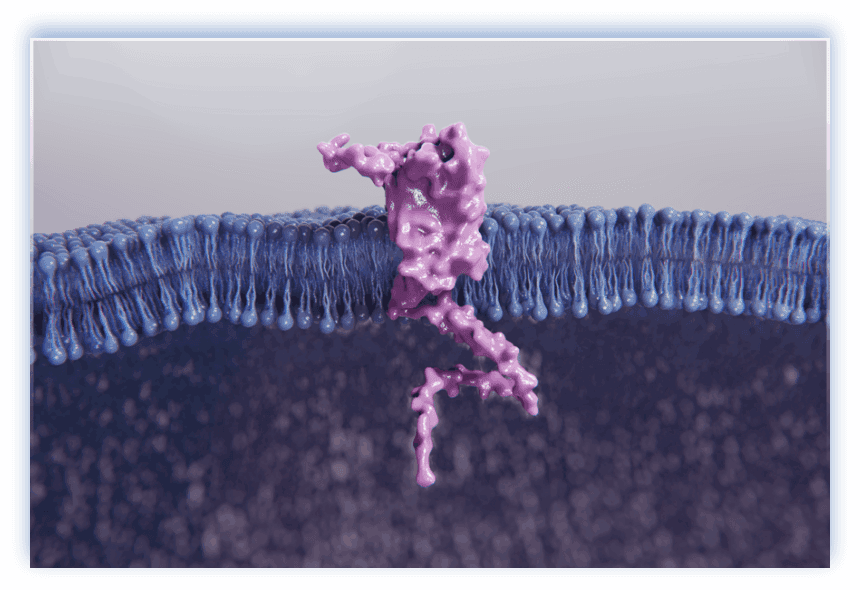
- GPRC5D, a transmembrane receptor protein with a short extracellular domain40-42
- Highly expressed on multiple myeloma cells, but minimal expression in healthy tissues40-42
- Expressed in a broad range of patients with MM (low- and high-risk)43
GPRC5D has CAR-T and bispecific antibody constructs under investigation40,44
FcRH537-39
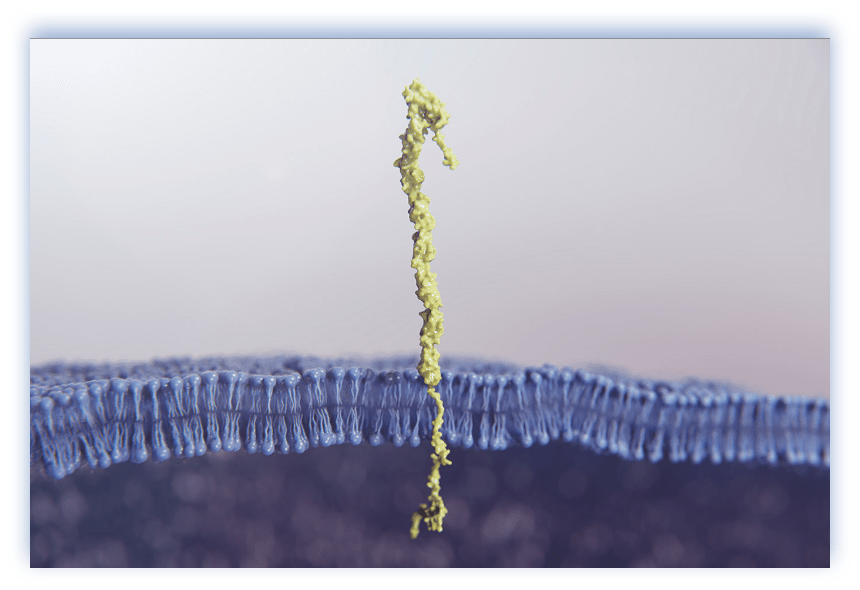
- FcRH5 is a cell surface antigen belonging to the immunoglobulin superfamily (IgSF)29,34
- Highly expressed on multiple myeloma cells compared to normal plasma cells29,34
- Expression reported in several B cell malignancies, suggesting broader applicability in hematological malignancies34,45
FcRH5 has ADC and bispecific antibody constructs under investigation29,40,46
ADC, antibody-drug conjugate; BCMA, B cell maturation antigen; CAR-T, chimeric antigen receptor T; CD, cluster of differentiation; CI, confidence interval; CRAB, hypercalcemia, renal insufficiency, anemia, bone/skeletal-related events; FcRH5, Fc receptor-homolog 5; GPRC5D, G-protein coupled receptor family C group 5 member D; MM, multiple myeloma; MOD, mechanism of disease; MRI, magnetic resonance imaging; NKG2D, natural killer group 2D; Ras, rat sarcoma virus; SINE, selective inhibitors of nuclear export; SLAMF7, signaling lymphocytic activation molecule family; SLiM, sixty percent or more clonal bone marrow plasma cells, light chains, MRI.
Extending the time between remissions and delaying disease progression remain important unmet needs in relapsed or refractory multiple myeloma. This cycle of relapse and remission highlights the constant need to explore new targets.18
Sign up to receive more information from Janssen Oncology
Sign Up NowReferences: 1. Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. 2. What is multiple myeloma? American Cancer Society. Updated February 28, 2018. Accessed April 3, 2023. https://www.cancer.org/cancer/multiple-myeloma/about/what-is-multiple-myeloma.html 3. Cancer facts & figures 2023. American Cancer Society. Accessed April 26, 2023. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html. 4. Cancer Stat Facts: Myeloma. National Cancer Institute. Accessed April 26, 2023. https://seer.cancer.gov/statfacts/html/mulmy.html 5. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. 6. Barwick BG, Gupta VA, Vertino PM, Bolse LH. Cell of origin and genetic alterations in the pathogenesis of multiple myeloma. Front Immunol. 2019;10:1121. 7. Manier S, Salem KZ, Park J, et al. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14(2):100-113. 8. Rajkumar SV, Landgren O, Mateos MV. Smoldering multiple myeloma. Blood. 2015;125(20):3069-3075. 9. Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590. 10. Minnie SA, Hill GR. Immunotherapy of multiple myeloma. J Clin Invest. 2020;130(4):1565-1575. 11. Borrello I. Can we change the disease biology of multiple myeloma? Leuk Res. 2012;36 Suppl 1(01):S3–12. 12. Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867-874. 13. Paccagnella A, Chiarion-Sileno V, Soesan M, et al. Second and third responses to the same induction regimen in relapsing patients with multiple myeloma. Cancer. 1991;68(5):975-980. 14. Drewinko B, Alexanian R, Boyer H, Barlogie B, Rubinow SI. The growth fraction of human myeloma cells. Blood. 1981;57(2):333-338. 15. Delforge, M. Treatment of relapsed multiple myeloma. In: Mohty M, Harousseau J-L, eds. Handbook of Multiple Myeloma. Springer International Publishing; 2015:65-77. https:doi.org/10.1007/978-3-319-18218-6_5. 16. Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120(5):1067-1076. 17. Sonneveld P. Management of multiple myeloma in the relapsed/refractory patient. Hematology Am Soc Hematol Educ Program. 2017;(1):508-517. 18. Mikhael J. Treatment options for triple-class refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(1):1-7. 19. Usmani S, Ahmadi T, Ng Y, et al. Analysis of real-world data on overall survival in multiple myeloma patients with ≥3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016;21(11):1355-1361. 20. Gandhi UH, Cornell RF, Lakshman A, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33(9):2266-2275. 21. Mateos M-V, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. 2022;36(5):1371-1376. 22. Franssen LE, Mutis T, Lokhorst HM, van de Donk NWCJ. Immunotherapy in myeloma: how far have we come? The Adv Hematol. 2019;10:1-19. 23. Ishibashi M, Soeda S, Sasaki M, et al. Clinical impact of serum soluble SLAMF7 in multiple myeloma. Oncotarget. 2018;9(78):34784-34793. 24. Parikh K, Cang S, Sekhri A, Liu D. Selective inhibitors of nuclear export (SINE)–a novel class of anti-cancer agents. J Hematol Oncol. 2014;7:78. 25. Strassl I, Schreder M, Steiner N, et al. The agony of choice—where to place the wave of BCMA-targeted therapies in the multiple myeloma treatment puzzle in 2022 and beyond. Cancers (Basel). 2021;13(18):4701. 26. Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14(9):2775-2784. 27. Tai Y-T, Landesman Y, Acharya C, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia. 2014;28(1):155-165. 28. Carpenter RO, Evbuomwan MO, Pittaluga S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19(8):2046-2060. 29. Stewart AK, Krishnan AY, Singhal S, et al. Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma. Blood Cancer J. 2019;9(2):17. 30. Morandi F, Horenstein AL, Costa F, et al. CD38: A target for immunotherapeutic approaches in multiple myeloma. Front Immunol. 2018;9:2722. 31. Dhakal B, Hari PN, Usmani SZ, Hamadani M. Chimeric antigen receptor T cell therapy in multiple myeloma: promise and challenges. Bone Marrow Transplant. 2020;56(1):9-19. 32. Rodríguez-Otero P, Prósper F, Alfonso A, Paiva B, San Miguel JF. CAR T-cells in multiple myeloma are ready for prime time. J Clin Med. 2020;9(11):3577. 33. Timmers M, Roex G, Wang Y, et al. Chimeric antigen receptor-modified T cell therapy in multiple myeloma: beyond B cell maturation antigen. Front Immunol. 2019;10:1613. 34. Li J, Stagg NJ, Johnston J, et al. Membrane-proximal epitope facilitates efficient T cell synapse formation by anti-FcRH5/CD3 and is a requirement for myeloma cell killing. Cancer Cell. 2017;31(3):383-395. 35. Dose escalation study of talquetamab in participants with relapsed or refractory multiple myeloma. ClinicalTrials.gov. Published January 16, 2018. Updated March 24, 2023. Accessed April 3, 2023. https://clinicaltrials.gov/ct2/show/NCT03399799. 36. Dose-escalation study of cevostamab in participants with relapsed or refractory multiple myeloma (R/R MM). ClinicalTrials.gov. Published September 7, 2017. Updated February 21, 2023. Accessed April 3, 2023. https://www.clinicaltrials.gov/ct2/show/NCT03275103. 37. Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583-589. 38. Varadi M, Anyango S, Deshpande M, et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50(D1):D439-D444. 39. RCSB PDB. Computed structure model of Fc receptor-like protein 5. Updated September 30, 2022. Accessed April 3, 2023. https://www.rcsb.org/structure/AF_AFQ96RD9F1. 40. Cho SF, Xing L, Anderson KC, Tai YT. Promising antigens for the new frontier of targeted immunotherapy in multiple myeloma. Cancers (Basel). 2021;13(23):6136. 41. Pillarisetti K, Edavettal S, Mendonça M, et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135(15):1232-1243. 42. Verkleij CPM, Broekmans MEC, van Duin M, et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021;5(8):2196-2215. 43. Atamaniuk J, Gleiss A, Porpaczy E, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur J Clin Invest. 2012;42(9):953-960. 44. Smith EL, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485). doi: 10.1126/scitranslmed.aau7746. 45. Ise T, Nagata S, Kreitman RJ, et al. Elevation of soluble CD307 (IRTA2/FcRH5) protein in the blood and expression on malignant cells of patients with multiple myeloma, chronic lymphocytic leukemia, and mantle cell lymphoma. Leukemia. 2007;21(1):169-174. 46. Elkins K, Zheng B, Go M, et al. FcRL5 as a target of antibody–drug conjugates for the treatment of multiple myeloma. Mol Cancer Ther. 2012;11(10):2222-2232.